Our Pipeline
NEUROLOGY
Project code
(INN)
(INN)
Indication
Preclinical
Phase 1
Proof-of-Concept
Pivotal
BLA/NDA Review
Partners
HL192 (Nurr-1 Activator)
Parkinson's Disease (PD)

OPHTHALMOLOGY
Project code
(INN)
(INN)
Indication
Preclinical
Phase 1
Proof-of-Concept
Pivotal
BLA/NDA Review
Partners
HL036 (tanfanercept)
Dry eye disease (DED)


IMMUNOLOGY
Project code
(INN)
(INN)
Indication
Preclinical
Phase 1
Proof-of-Concept
Pivotal
BLA/NDA Review
Partners
Batoclimab
Myasthenia gravis (gMG)


Batoclimab
Thyroid eye disease (TED)

Batoclimab
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)

Batoclimab
Graves' Disease (GD)

Imeroprubart
Cutaneous Lupus Erythematosus (CLE)

Imeroprubart
Sjögren's disease (SjD)

Imeroprubart
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP)

Imeroprubart
Myasthenia gravis (gMG)

Imeroprubart
Difficult-To-Treat Rheumatoid Arthritis (D2T RA)

Imeroprubart
Grave's disease (GD)

HL192 (ATH-399A)
HL192 (NurrOn Code name: ATH-399A) is a pipeline candidate originated from NurrOn Pharmaceuticals, targeting Nurr-1 for the development and maintenance of dopaminergic neurons. This compound has the potential to slow the progression of Parkinson's disease (PD) by activating Nurr1. Nurr1 activators enhance the contrasting dual functions of Nurr1 by increasing transcriptional activation of dopaminergic-specific genes and further enhancing transrepression of neurotoxic proinflammatory gene expression in microglia. Notably, these Nurr1 activators have shown significant improvement in behavioral deficits in both 6-hydroxydopamine lesioned rat and MPTP-lesioned mouse models of PD without any detectable signs of dyskinesia-like behavior. HL192 is currently being developed for the treatment of various neurodegenerative diseases, including PD.
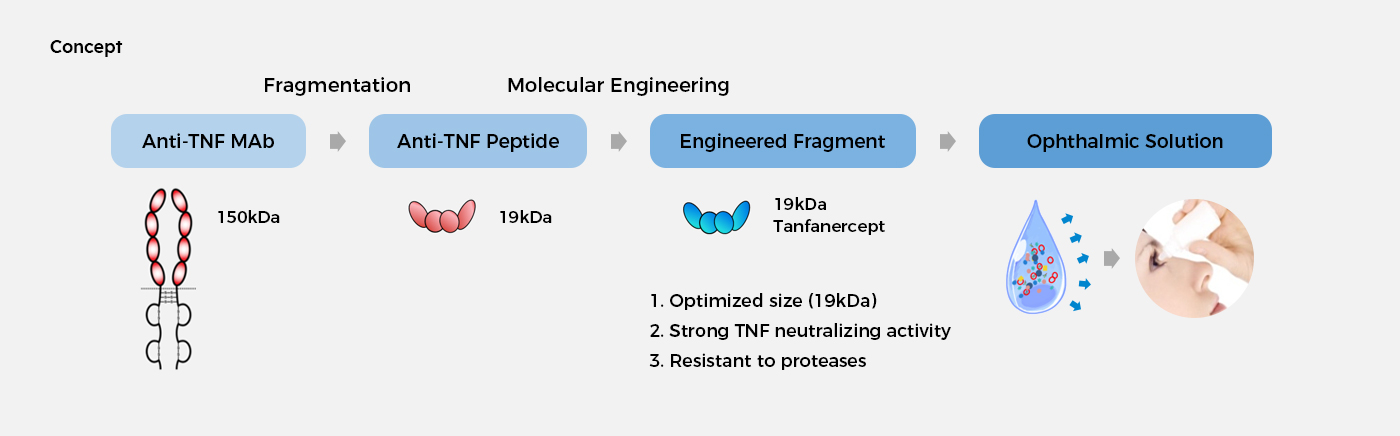
Tanfanercept (HL036)
Tanfanercept is the most advanced novel biological approach to suppressing tumor necrosis factor (TNF) that causes inflammation in the body. HanAll is developing tanfanercept as an ophthalmic drug to provide better treatment options to the patients while extending the indications to relevant diseases.
Mechanism of Action
Tumor necrosis factor is a pro-inflammatory cytokine that plays a pivotal role in inflammatory response. Tumor necrosis factor receptor 1 (TNFR1) signaling is known to be pro-inflammatory and the suppression of TNFR1 is crucial for the treatment of autoimmune diseases. Tanfanercept is a molecularly engineered TNFR1 fragment adopting ’Resistein’, an amino acid substitution technique originally developed by HanAll Biopharma. Tanfanercept strongly binds to TNF and exhibits high stability, as it is resistant to enzymatic lysis. In addition, tanfanercept is efficiently distributed across the whole body with local administration owing to its small size (approximately 19 kDa). Based on these characteristics, we expect that tanfanercept will be effective in suppressing inflammatory responses, and improving symptoms of inflammatory eye disease.
HL161(Batoclimab, Imeroprubart)
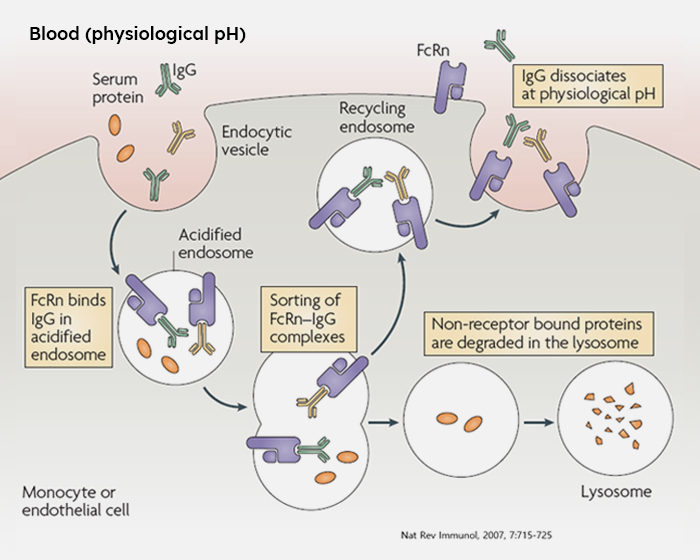
HL161(Batoclimab, Imeroprubart) is a novel, fully human monoclonal antibody targeting the neonatal Fc receptor (FcRn) which is known to increase the half-life of Immunoglobin G (IgG). Our goal is to cure multiple autoimmune diseases caused by pathogenic autoantibodies through the application of batoclimab, which decreases IgG levels in the body. HanAll Biopharma is attempting to expand indications of batoclimab to a number of autoimmune diseases without appropriate treatments. We are hoping to contribute to the patients’ quality of life by developing batoclimab as a subcutaneous injection for the convenience of both patients and clinicians.
Mechanism of Action
The neonatal Fc receptor (FcRn) is a membrane receptor usually found on the cellular surface that plays a role in preventing the degradation of immunoglobulin G (IgG) and serum albumin. FcRn binds to the IgG antibodies at the cellular surface and guides their transport through the cell, blocking their degradation in the endosome before getting released back into circulation. Batoclimab is designed to specifically bind to and inhibit FcRn, thus expediting the degradation of IgG antibodies and decreasing the levels of IgG antibodies in the systemic circulation.